

The spectrum of the heated gas was discrete lines or spectra lines.
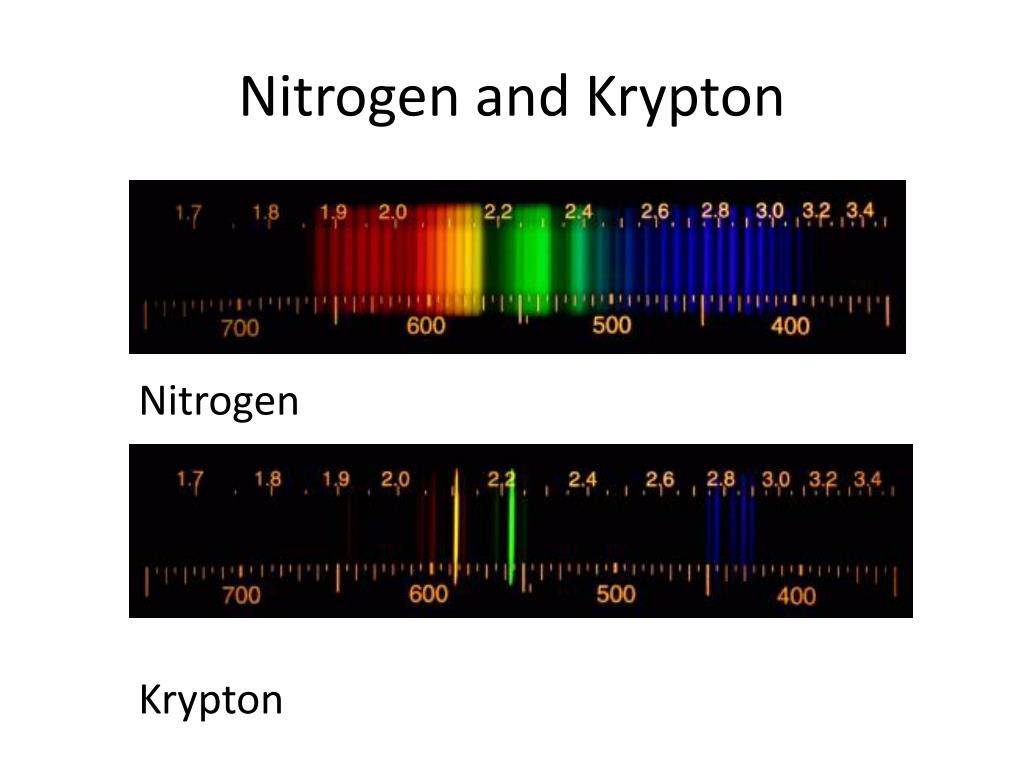
In 1850s, Anders Jonas Angstrom observed that the light emitted by a high heated gas did not have a continuum spectrum, like white light, when it passes through a prism. This was the beginning of the Spectrum Analyze Technique. In the 19 th century, scientist discovered that they could use the light emitted by heated or electrical discharged materials to analyze their properties. The table below shows the range of wavelength for each of seven colors of the rainbow. The result is that red light bends less sharply than violet as it passes through the prism, creating a spectrum of colors. The decomposition of the white light in different colors results from different wavelengths, as a consequence, they move at different speeds in the prism, with red light moving faster than violet. This means that the white light is formed by the combination all visible colors. In sequence of this experiment, Newton combined those colored beams in another prism which resulted in another white light beam. Newton classified this spectrum in a range of seven different colors (Red, Orange, Yellow, Green, Bleu, Indigo and Violet).
In the 1670s, Isaac Newton, during optical experiments observed that a beam of white light was decomposed in a continuum spectrum of all visible colors, like a rainbow, when it pass through a prism.


 0 kommentar(er)
0 kommentar(er)
